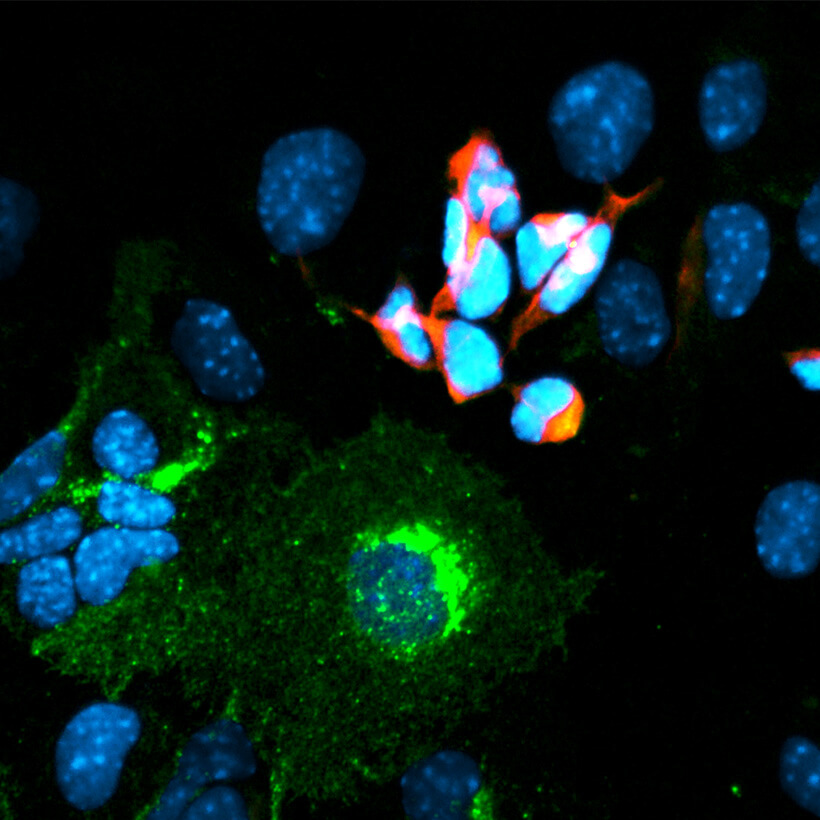
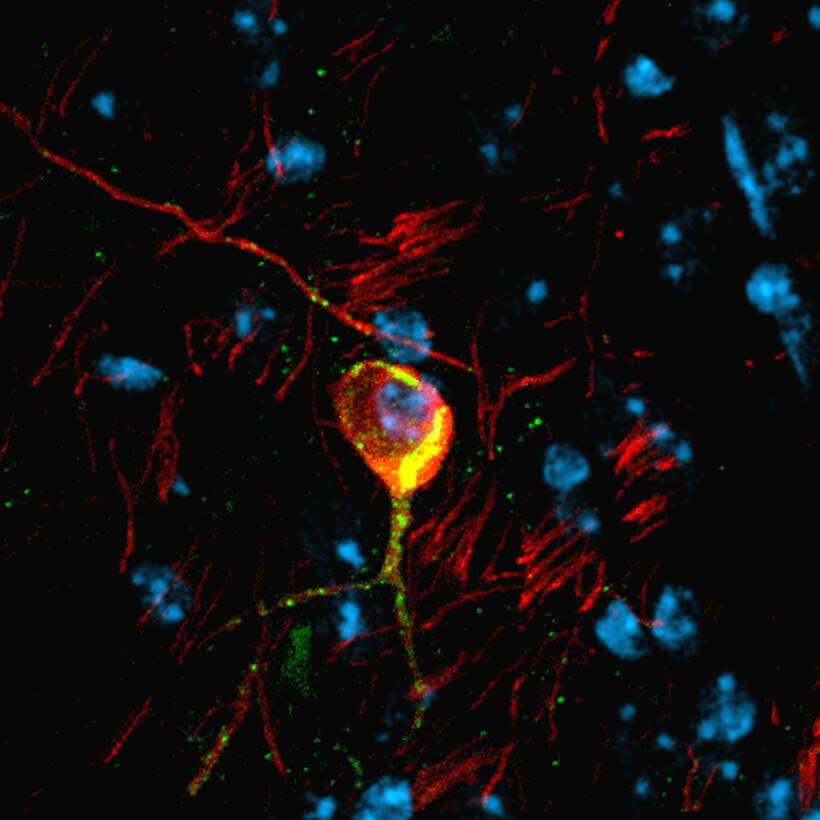
RESEARCH
The discovery of neural stem cells (NSCs) in the adult central nervous system (CNS) implied the potential for endogenous repair and exogenous cell-based therapeutics. Although we foresee these potential applications of NSCs as a long-term consequence, determining positive and negative regulators that work to provide an optimal number of NSCs and specific cell types is crucial for their future use in pathological conditions.
Most genes required for mammalian development are expressed from both maternally and paternally inherited chromosomal homologues, however there are a small number of genes known as “imprinted genes” that only express a single allele from one parent, which is repressed on the gene from the other parent. Imprinted genes are dependent on epigenetic mechanisms such as DNA methylation and post-translational modifications of the DNA-associated histone proteins to establish and maintain their parental identity. In the brain, multiple transcripts have been identified which show parental origin-specific expression biases. Disruption of imprinting can result in a number of human imprinting syndromes such as Prader-Willi (PWS) and Angelman (AS) and is one of the most abundant alterations in cancer making aberrations of imprinting a potentially valuable tool for both diagnosis and treatment.
Our main focus of research is the study of the epigenetic mechanisms that regulate dosage control of imprinted genes in the neural stem cell pool and their contribution to physiology and pathology of the adult brain.
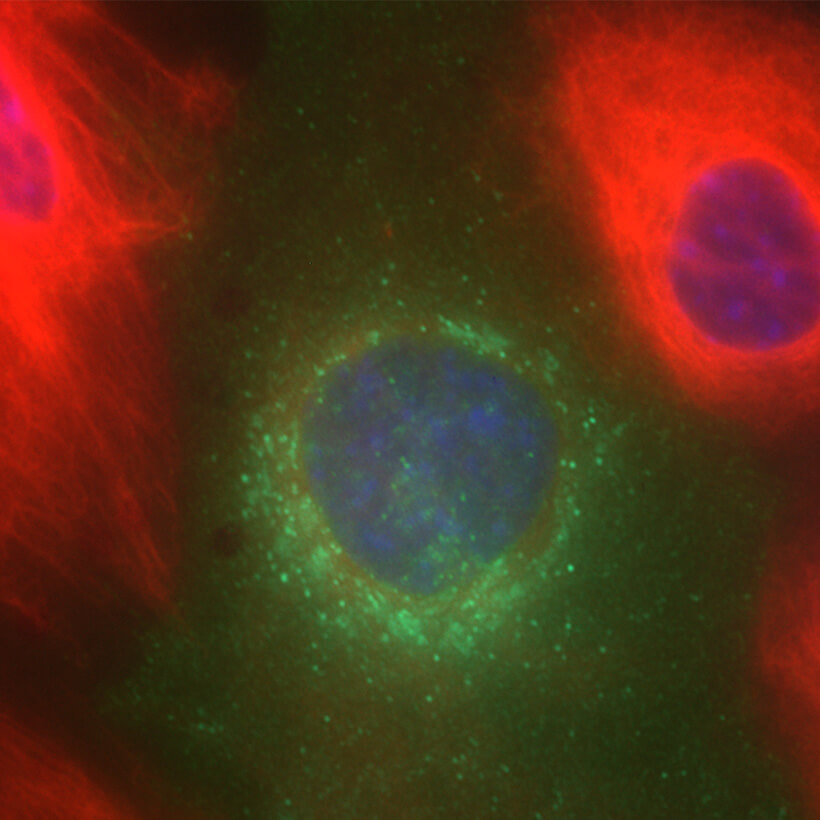
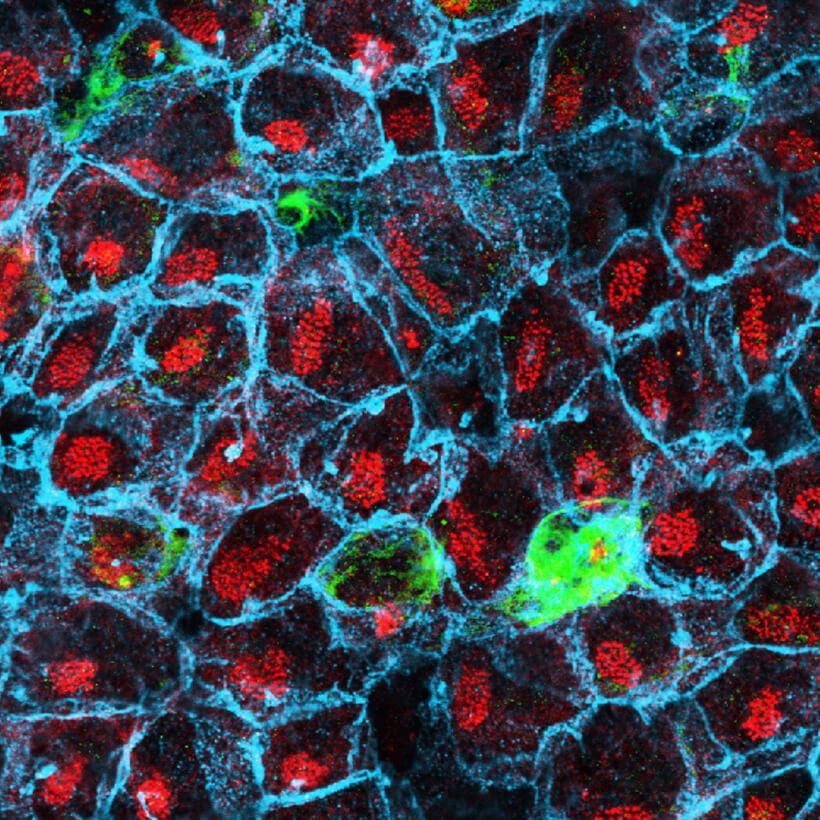

We could subdivide our research focus into the following specialized areas:
- Genomic imprinting in NSCs. The main line of the group has been focused on the understanding of genomic imprinting regulation in the adult neural stem cell pool and whether relaxation of imprinting might be an extended process in normal tissue stem cells.
- Epigenetic regulation of imprinted genes in NSCs. There is growing evidence suggests that epigenetic mechanisms play important roles in mammalian adult neurogenesis regulating gene expression and also through the modulation of high order chromatin architecture. We are interested in understanding, the precise molecular mechanisms and critical functional players of DNA methylation and demethylation and histone modifications that regulate imprinting control regions during specification and activation of adult NSCs
- Aberrations of genomic imprinting in the neurogenic niches: Imprinted genes play an important role in development and placental biology. Furthermore, as dosage of imprinted genes is crucial, disruption of imprinting can result in a number of human imprinting syndromes and may predispose to cancer by promoting tumorigenic or suppressing anti-tumor mechanisms. Indeed, an early occurring aberration in cancer is loss of imprinting (LOI). One important line of the group is the identification of genomic imprinting aberrations during the formation of brain tumors paying special attention to its epigenetic regulation such as distribution of methylation among and within these imprinted clusters.